Last edit: 17/07/2025
The explosion of dust is quite simple to imagine in terms of everyday experience. Any solid material that can burn will do so with an intensity and speed that increases as the fragmentation of the material increases. A piece of wood, when ignited, will burn slowly, releasing heat over a long period of time. If, on the other hand, it is cut into small pieces, the speed of combustion increases because the total contact surface between the wood and the air is greater, making it easier to ignite the wood.
If the breakdown continues down to particles of the order of a tenth of a millimetre or less in size, and the particles are suspended in a sufficiently large volume of air to give them sufficient space to burn without restriction, the burning rate will be very high and the energy required for ignition very small. Such a cloud is called a dust explosion.
The materials that can give rise to a dust explosion can be:
- – Natural organic materials (flour, wood, cloth, sugar, etc…)
- – Synthetic organic materials (plastics, organic pigments, pesticides, pharmaceutical compounds, etc.)
- – Coal and peat
- – Metals (aluminium, magnesium, titanium, zinc, iron, etc.)
The density of the powder for the blend to be explosive
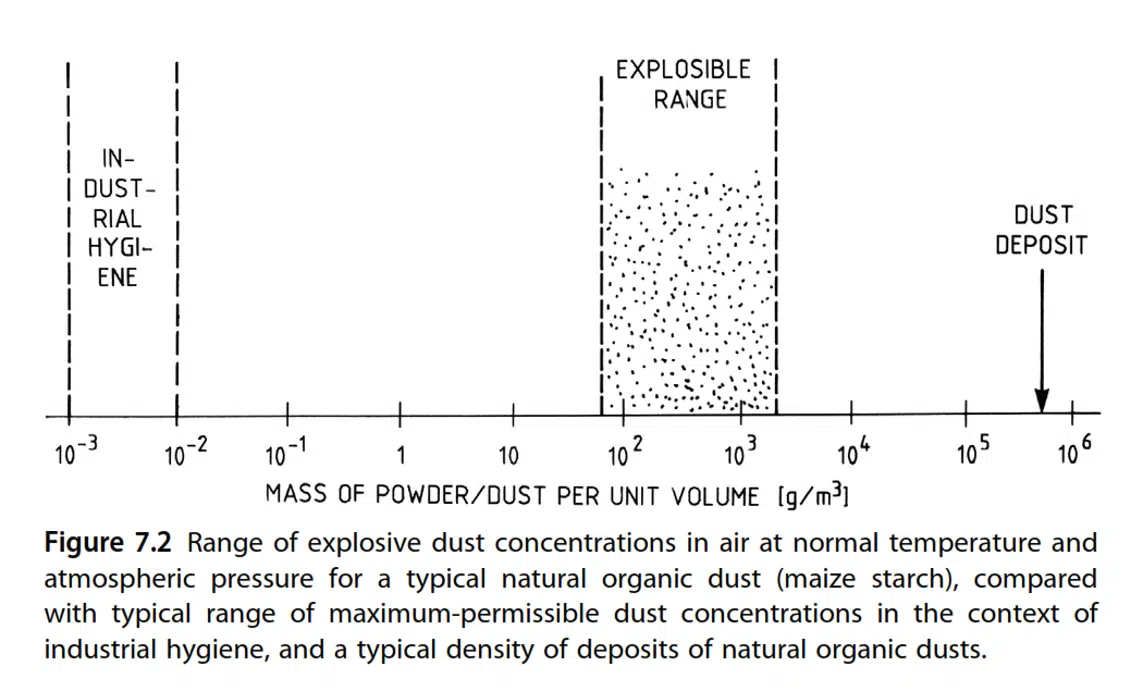
Fig. 7.2 shows the explosion range for a typical natural organic material, such as cornflour, in air at room temperature and atmospheric pressure. This range is rather narrow, extending over less than two orders of magnitude (from 100 g/m3 to 2 kg/m3).
The explosive limits differ between the various dusts. For example, zinc dust has a lower explosive limit of about 500 g/m3.
Explosive dust clouds have a high optical density, even at the LEL. This is demonstrated, as in Fig. 7.2, by the fact that the range of maximum permissible dust concentrations, which are specified in the context of workplace cleaning, is three or four orders of magnitude lower than the minimum explosive dust concentrations. This means that the unpleasant levels of dust concentration that can occur in the working environment of an establishment, which are checked by the authorities, are far lower than the concentration levels that can spread the fire.
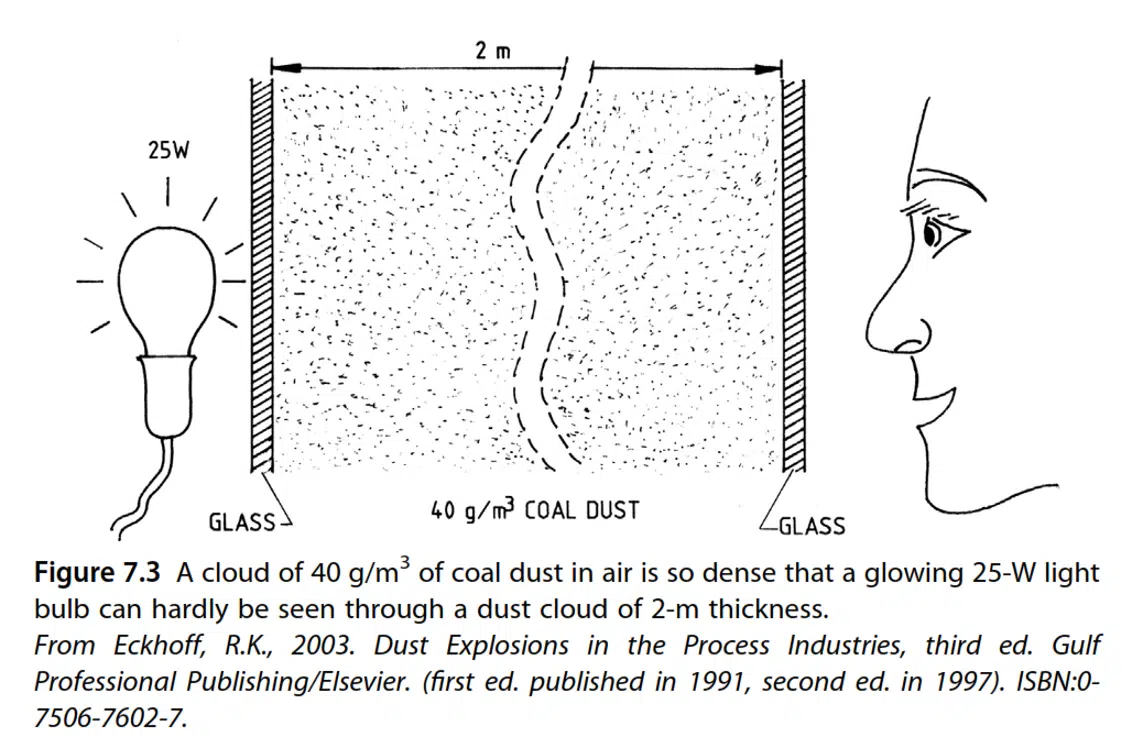
Consequently, the minimum explosive concentration (MEC or mass LEL) corresponds to a dust cloud with a high optical density, which is unlikely to occur regularly in establishments. Fig. 7.3 shows the high optical densities of explosive dust clouds, based on a rule of thumb from Intelmann and quoted by Zehr (1965): “Consider a 25 W bulb observed from 2 m away in the dust, if the bulb is not visible, the concentration is more than 40 g/m3, i.e., about half the MEC”.